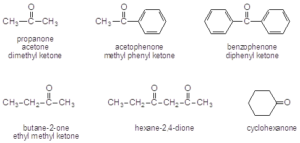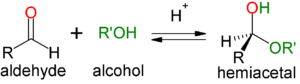Aldehydes and Ketones I: Electrophilicity and Oxidation-Reduction section provides High Yield information for College Students, Medical Students to succeed in the MCAT exam and Medical School.
Table Of Contents
Aldehydes and Ketones I: Description and Properties
- Ketone: two alkyl groups bonded to the carbonyl
- Aldehyde: one alkyl group and one hydrogen
- Characterized by strong smell (found in many spices)
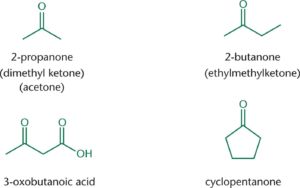
Nomenclature
- Aldehydes are named by replacing the –e at the end of an alkane with the suffix –al
- When named as substituents, use the prefix oxo-
- Common names for first five aldehydes:
- -formaldehyde
- -acetaldehyde
- -propionaldehyde
- -butyraldehyde
- -valeraldehyde
- If an aldehyde is attached to a ring, the suffix –carbaldehyde is used
- Ketones are named by replacing the –e with the suffix –on
- When naming ketones by common name, the two alkyl groups are named alphabetically followed by –ketone.
- When names as substituents, use either oxo- or keto-
-


Physical Properties
- Governed by the presence of the carbonyl group. Dipole is stronger than the dipole of an alcohol since the double-bonded oxygen is more electron-withdrawing.
- Even though aldehydes and ketones have dipoles that are more polar, the boiling point of alcohols is higher due to the presence of hydrogen bonding in an alcohol.
- Both usually act as electrophiles due to the electron withdrawing properties of the carbonyl oxygen, which leaves a partial positive charge on the oxygen.
- Generally, aldehydes are more reactive towards nucleophiles since they have less steric hindrance and fewer electron donating groups than ketones.
Formation
- An aldehyde can be formed from the partial oxidation of a primary alcohol, but only with PCC.
- A ketone can be obtained from the oxidation of a secondary alcohol. Range of reagents can be used (from dichromate, chromium trioxide to PCC).
- No concern for oxidizing too far since the reactants will stop at the ketone stage.
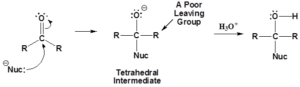
Nucleophilic Addition Reactions
- Carbonyl carbon has a partial positive charge and carbonyl oxygen has a partial negative charge, which make it prime for a nucleophilic attack.
- Nucleophile forms a covalent bond to the carbon which break the pi bond associated with carbonyl.
- The electrons from the pi bond are pushed onto the oxygen atom.
- This bond breaking forms a tetrahedral intermediate
- Any time a carbonyl is opened, should ask: Can I reform the carbonyl?
- Carbonyl will not reform if no good leaving group is present (aldehydes/ketones)
- O– will simply accept a proton form the solvent to form a hydroxyl group (alcohol)
- Carbonyl double bond reforms if good leaving groups are present (carboxylic acid and its derivatives)
- Double bond pushes off the leaving group

- Carbonyl will not reform if no good leaving group is present (aldehydes/ketones)
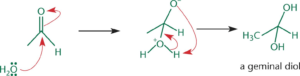
Hydration
- In presence of water, aldehydes/ketones react to form germinal diols (1,1- diols)
- Nucleophilic oxygen in water attacks the electrophilic carbonyl carbon
Acetals and Hemiacetals
- Hemiacetal: when one equivalent of alcohol (acts as the nucleophile) is added to an aldehyde
- Hemiketal: when one equivalent of alcohol (acts as the nucleophile) is added to a ketone
- Recognized by the retention of the hydroxyl group
- Hemi is known as the halfway point and is the endpoint in basic conditions
- When two equivalents of alcohol are added, the reaction proceeds to completion which results in the formation of an Acetal and a ketal.
- Reaction proceeds by the substitution reaction SN1 and is catalyzed by anhydrous acid
- Hydroxyl group of a hemiacetal or hemiketal is protonated under acidic conditions and lost as a molecule of water. Which leads to the formation of a carbocation.
- Another equivalent of alcohol attacks the carbocation which results in the formation of an Acetal or ketal.
- Are relatively inert, so they are often used as protecting groups for carbonyl functionalities.
- Can be converted back to carbonyls with aqueous acid and heat

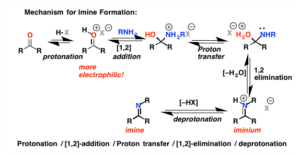
Imines and Enamines
- Nitrogen and nitrogen based functional groups act as good nucleophiles since nitrogen has a lone pair of electrons
- Imine: simplest case. Ammonia adds to a carbon atom and water is lost
- Compound with a nitrogen atom double bonded to a carbon atom
- Condensation reaction: small molecule is lost in the formation of the bond
- Nucleophilic substitution since nitrogen replaces carbonyl oxygen
- Common ammonia derivatives: hydroxylamine, hydrazine, semicarbazide
- Enamines: contain both a double bond and a nitrogen-containing group
- Imines can undergo tautomerization to form these

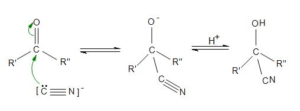
Cyanohydrins
- Hydrogen cyanide is a classic nucleophile on the MCAT
- Has both triple bonds and an electronegative nitrogen atom which makes it relatively acidic (pKa of 9.2).
- After the hydrogen atom dissociates, the nucleophilic CN– can attack the carbonyl carbon atom.
- Reaction with aldehydes and ketones produces stable compounds called cyanohydrins
- Gains stability from newly formed C-C bond.

Oxidation-Reduction Reactions
- Aldehydes are in the middle of the redox spectrum since they are more oxidized than alcohols but less oxidized than carboxylic acid
- Ketones are as oxidized as secondary carbons can get.
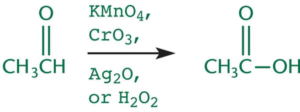
Oxidation of Aldehydes
- When aldehydes are oxidized further they from carboxylic acid.
- Facilitated through any oxidizing agent that is stronger than PCC
- Facilitated through any oxidizing agent that is stronger than PCC
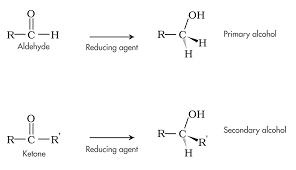
Reduction by Hydride Reagents
- Can also undergo reduction to form alcohols
- Often performed by hydride reagents (lithium aluminum hydride & sodium borohydride)